Features
- Air flow rate from 50 to 30000 CFM
- Material of construction: PP, PP+FRP, MS, SS 304, SS 316
- Scrubbing efficiency up to 99 %Lower shell serves as integral sump for recycle of liquid
- Spray nozzles accessible from top of unit
- Entrainment separators prevent liquid carryover
- Special multi-bed packing designs accommodate different scrubbing solutions for removal of multiple contaminants
Highlights
- Our veteran design team is focused on specialized solutions built around your unique requirements.
- A range of designs and approaches are available for Wet Scrubbers, but all generally incorporate a system fan, recycle pump, mist eliminator, exhaust stack and instrumentation & controls. Our systems are comprehensive in nature, including all necessary equipment to function as a complete solution.
- We thrive on unique challenges and want to be your provider for all industrial needs relating to air pollution equipment.
- We can design and manufacture a custom Wet Sulfuric Acid Scrubber to fit your precise application and will provide you with outstanding performance and low maintenance requirements. Contact us with your process information so we can begin discussing the type of new equipment that best fits your situation.
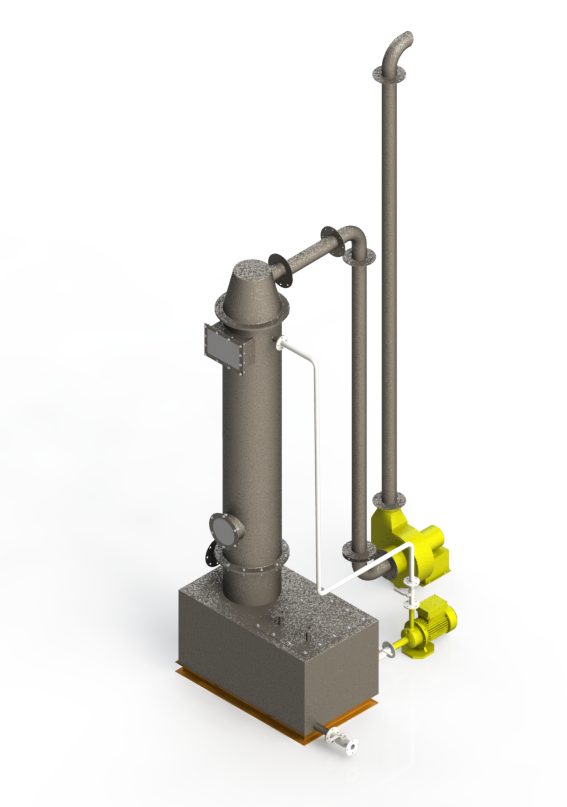
Mechanism
Sulfuric acid (H2SO4) scrubber
- Chemical producers that emit sulfur-based substances such as sulfuric acid or sulfur dioxide as part of their manufacturing processes are under strict requirements to remove these toxic byproducts from their emissions.
- Drizgas Tech is one of leading manufacturer of sulfuric acid scrubber in India. We provide highly efficient, top-quality Sulfuric Acid Scrubber systembuilt around your precise requirements.
- Sulfuric acid wet Scrubber systems are a diverse group of air pollution control devices that can be used to remove gases from industrial exhaust streams. Traditionally, the term scrubber refers to pollution control devices that use liquid to wash unwanted pollutants from a gas stream.
Sulfuric acid
Sulfuric acid is a highly corrosive substance that can damage and destroy human tissues. Its vapor, however, is strongly irritant to the respiratory tract and can cause severe pulmonary edema which could prove fatal.
Exposure limits
OSHA: The legal airborne permissible exposure limit (PEL) : 1 mg/m3 (8 hour average)
NIOSH: The recommended airborne exposure limit (REL): 1 mg/m3 (10 hour average)
ACGIH: The threshold limit value (TLV): 0.2 mg/m3
Mechanism
- Wet Scrubbers remove pollutants by injecting liquid into the gas stream. The scrubber system functions as follows: The liquid introduced into the gaseous waste stream causes chemical reactions that convert the pollutant(s) into a different chemical compound.
- Many Wet Sulfuric Acid Scrubbers utilize a multi-stage approach, first using a bed of packed media with recirculated liquid caustic (sodium hydroxide solution) which acts as a base to neutralize the acid, and finally uses a mist eliminator to remove water vapor.
- The recirculating scrubber and the packed column scrubber are the two scrubbing systems used most commonly. The Recirculating Scrubber consists of a pump, lines, eductor, scrubber tank, and sparger.
- In this system, the fume eductor draws acid vapors from the storage tank. The acid vapors are then absorbed by water being recirculated through the eductor.
- The solution in the scrubber tank gradually builds strength up to a maximum of 15% HCl. At this point, the weak acid solution should be removed for use or sent to an appropriate hazardous waste treatment site.
- The scrubber tank is then recharged with fresh water. No acid is lost and the fumes from the hydrochloric acid are contained by the system.
Chemistry of H2SO4 reaction with NaOH
A neutralization reaction is a type of double replacement reaction in which the reactants are an acid and a base, and the products are a salt and water (acid + base → salt + water). A salt is an ionic compound that results from a neutralization reaction between an acid and a base. The chemical equation for this reaction is:
H2SO4 + 2NaOH → Na2SO4 + 2H2O
Na2SO4 is the salt sodium sulfate.
Wet Sulfuric Acid Scrubbers can vary widely in terms of size and design. Factors must be considered, including:
- The amount and concentration of sulfuric acid in the waste stream
- The presence of other pollutants in the waste stream
- The process conditions such as exhaust temperature and flow rate
Applications
- Steel industries exhaust scrubbing
- Food manufacturing industries exhaust scrubber
- Coal industries exhaust scrubbing
- Chemical factory wet scrubbing
- Reactor vent scrubbing
- Process vent scrubbing
- Acid Plating emission scrubbing
- Acid etching emission scrubbing
- Storage room exhaust scrubbing
