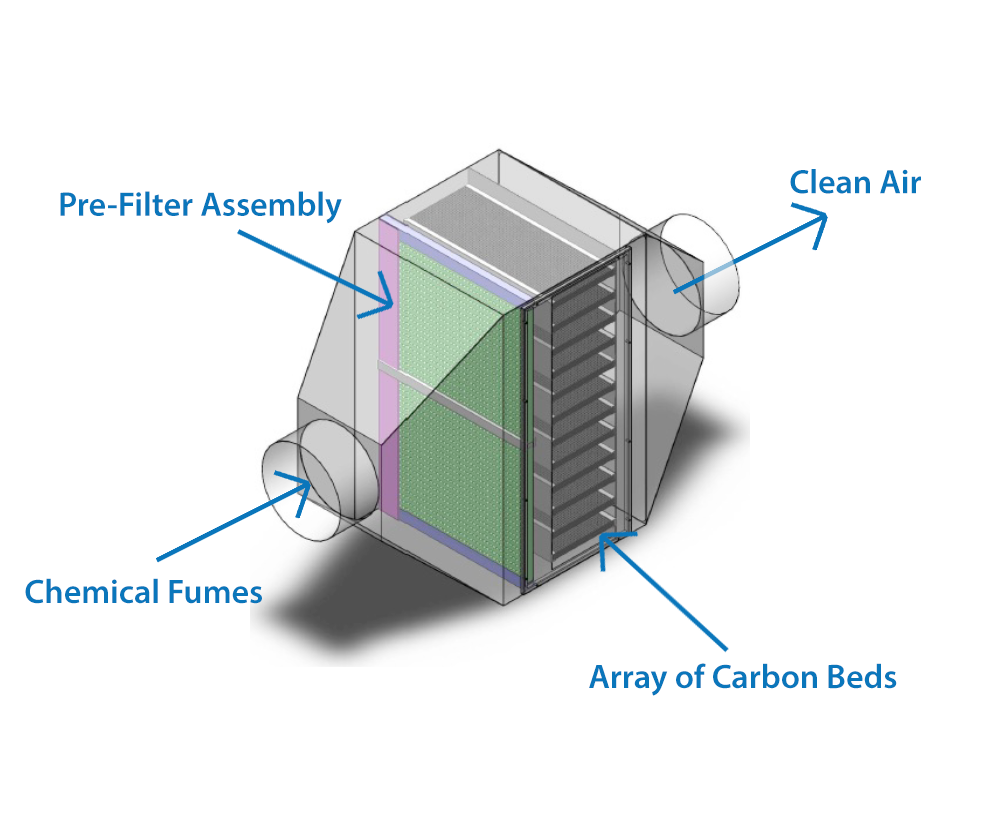
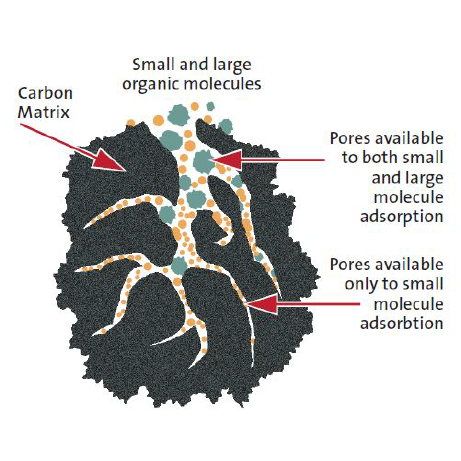
A dry scrubber, or dry scrubber system, is a type of pollution control equipment that is designed to remove harmful gases and particulates from industrial exhaust streams. Dry scrubbers are primarily used to abate solvent and acidic gases. This is one of the most common pieces of equipment found in manufacturing plants, because of its ability to handle less concentration of pollutant, highly acidic exhaust streams.
Working principle
A dry scrubber works by combining carefully chosen chemical reagents with the exhaust stream at incredibly high speed, which react with or adsorb the compounds in the stream. These chemicals react differently, depending on which compounds are being targeted; reactions either neutralize the dangerous Volatile organic compounds (VOCs) or adsorb them into a different compound all-together. Once converted, the substance left over can then be disposed of or transported easily. Each type of scrubber media removes particles and compounds in a different way; some chemical reactions, while others use electrostatic adhesion.
Features & Options
- Competitive packages including instrumentation and controls, skid mounting of equipment, acid storage tanks, metering pumps, and installation
- Vertical and horizontal configuration
- Efficiencies up to 99.9 %
- Capacities of 50 to 30,000 CFM
- Custom designed systems
- Options to be retrofitted with current equipment
- Media options
- Faster commissioning time
- In-house designed and manufactured control panels
- Sized system process fans
- Portable options and sizes available
Pollutants can be removed
- Solvents
- VOCs
- Ammonia
- Chlorine
- Hydrochloric Acid
- Chlorinated Silanes
- Metallic Compounds
- Sulfur Dioxide
- Hydrogen Sulfide
- Boron Trifluoride
- Amines
Why Choose a Dry Scrubber?
- Remove less concentrated pollutant in exhaust stream
- Versatile
- Capable of abating very acidic streams
- Spent media can be a source of revenue
- Smaller space requirements
- Can be retrofitted into current equipment
- Lower cost of purchase
- Able to neutralize highly corrosive gases
- Several customizable options, based on specific output and applications, often allowing for a reduction in cost.
- Eliminates as much or more than 99% of dangerous gases
- Doesn’t produce any wet sludge
- We can include a fabric filter to effectively control the particulates
Possible Disadvantages
- Disposal of waste and mediacan incur higher costs
- The chemicals collected may be too contaminated to be recycled in any way
Commonly used in following industries
- Asphalt processing
- Pharmaceuticals
- Landfills and Biogas
- Coaters
- Textile Processing
- Tar Removal
- Curing ovens
- Vinyl Manufacturing
- Oil and Gas
- Acid mist control
- Fertilizer Manufacturing
- Wastewater treatment
- Steel Processing
- Electronics
- Food processing
- Cocoa Processing
- Nuclear Waste filtration
- Printing
- Precious metal recovery
- Etching
- Wood Products